Cellular Injury
Fracture Biology
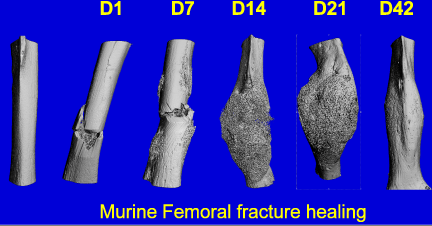
Oxygen tension in the environment surrounding bone cells can vary under certain circumstances. For example, skeletal unloading has been shown to induce osteocyte hypoxia. In addition, bone cells in the vicinity of a fracture experience a significant decrease in oxygen tension due to disruption of the vasculature. We are pursuing this line of study by investigating the effects of oxygen on bone cell differentiation, physiology and ultimately bone turnover. Additionally, we are investigating the influence of hypoxia on stem cell migration including trafficking of mesenchymal stem cells to regions of hypoxia.
Mechanisms of Post-Traumatic Osteoarthritis
By Blaine Christiansen
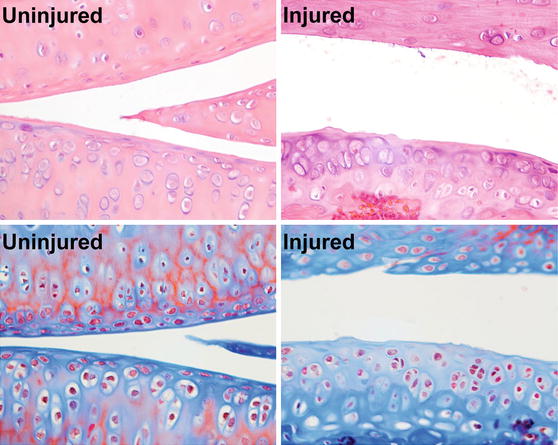
Post-traumatic osteoarthritis (PTOA) affects at least 50% of people who sustain a traumatic joint injury such as anterior cruciate ligament (ACL) rupture, with symptomatic joint pain typically developing within 1-2 decades following injury. The goal of this research is to determine the time course and mechanisms of PTOA progression and investigate therapies that can be applied at the time of injury that will slow or prevent the onset of PTOA. In particular, we are interested in examining the role of mechanical loading (exercise) or unloading (rest) and subchondral bone remodeling in the development of PTOA. We have developed a novel non-invasive mouse knee injury model that uses a single externally-applied mechanical load to rupture the ACL, inducing inflammation and biomechanical changes in the joint. We have characterized the time course of joint degeneration following non-invasive knee injury in mice, and have found that this model faithfully reproduces many of the key structural and biological changes of human OA, including inflammation, fibrosis, synovitis, and trabecular bone and cortical plate thinning at early time points post-injury, and articular cartilage degradation, osteophyte formation, and subchondral cortical bone plate thickening and sclerosis at terminal time points.
Our ongoing studies use this clinically relevant injury model (non-invasive, mechanically-induced ACL rupture in mice) and clinically relevant post-injury conditions (mechanical unloading of the injured limb, intermittent reloading, surgical restabilization of the joint) to determine the effect of biomechanical therapies for slowing PTOA progression after injury.
Mechanisms of Systemic Bone Loss Following Fracture
By Blaine Christiansen
Osteoporotic fractures account for two million broken bones in the United States each year, and identification of those at the greatest risk of fracture remains a challenge. Rather than age, hormone status, or other covariates, the most accurate predictor of future fracture is a previous fracture at any skeletal site, even after controlling for bone mineral density (BMD). The etiology of this relationship is unknown, but it is likely that fracture causes a systemic bone adaptation response that decreases both bone quantity and bone quality, thus actively compromising the entire skeleton.
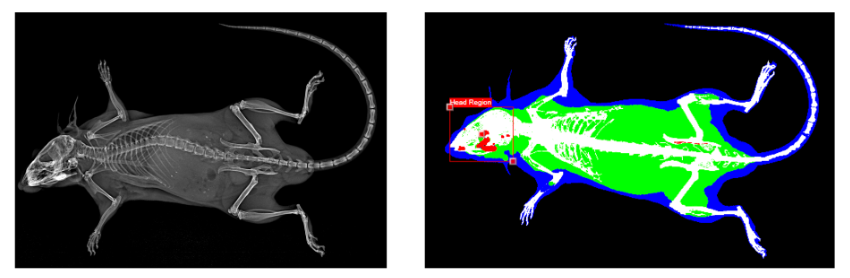
Data from our lab show that bone fracture and other injuries in mice actively decrease trabecular bone volume at distant skeletal sites within two weeks post-injury. However, a significant knowledge gap remains in identifying the individual contributions of processes such as injury-induced inflammation and decreased mechanical loading that could affect changes in bone quantity and bone quality after fracture. Analysis of these adaptive processes is a crucial step toward identifying therapeutic targets for treatment. The long-term goal of this research is to investigate mechanisms of systemic bone loss following fracture, and to translate these findings to human health and treatment of clinical fractures.
Our ongoing studies investigate changes in bone quantity and bone quality following fracture in mice, and will determine the dependence of these adaptive processes on factors such as injury-induced inflammation, decreased mechanical loading, age, and sex.
Contacts
Clare Yellowley
Professor
Blaine Christiansen
Associate Professor
Damian Genetos
Associate Professor
Benjamin Osipov
Post-Doc
