Musculoskeletal Transplant Immunology
While we can create well-functioning engineered cartilage, a significant challenge lies in predicting how the recipient's immune system will react upon implantation. Our research delves into the interaction between implants and the recipient's immune system. Our goal is to develop musculoskeletal tissues that are universally compatible from an immune perspective. This breakthrough would empower orthopedic surgeons to repair damaged tissues more effectively, without the concern of transplant rejection.
Canine Cartilage Tissue Engineering Using Surgical Waste of Osteochondrosis Dissecans Fragments as a Novel Cell Source
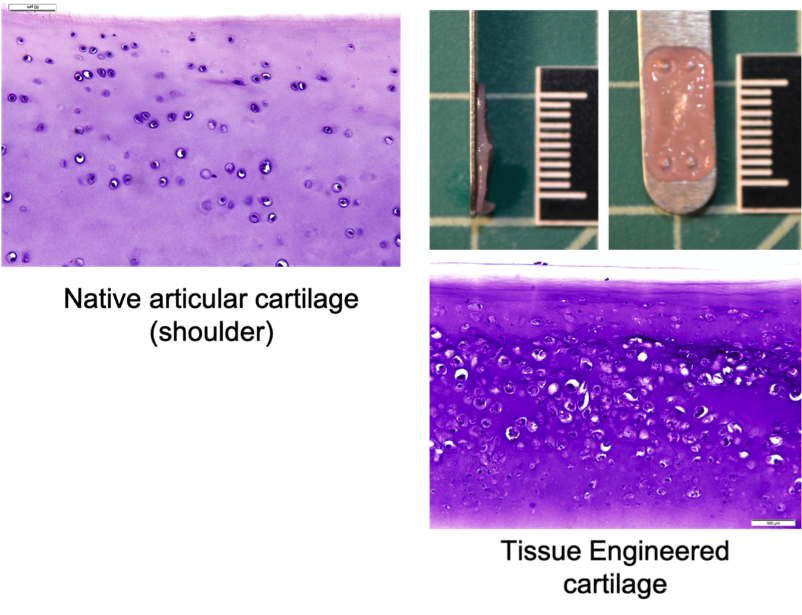
Osteochondrosis Dissecans (OCD) is a painful orthopedic disorder that typically affects juvenile dogs of large breeds. In this condition, a portion of the cartilage covering the articulating surfaces of the bones, such as the shoulder and knee, is detached. The detached cartilage no longer serves the purpose of shock absorption, which results in pain and discomfort. If left untreated, the condition gradually progresses to osteoarthritis. The most popular treatment is the surgical removal of the OCD cartilage (debridement). The debrided piece of OCD cartilage is then discarded, and the cartilage defect is left to heal through scar formation. The scar tissue provides temporary pain relief, and some dogs may return to full function. However, with time, the scar tissue degrades, and osteoarthritis resumes with age.
At the VORL, we are working on using the discarded OCD cartilage as a novel cell source for engineering new cartilage tissue. This process is called tissue-engineering. Tissue-engineered cartilage needs to be robust and durable to provide long-term replacement tissue for canine patients. Our team is currently working on improving the functional properties of engineered cartilage to bring it closer to clinical implantation. We are also working on devising novel methods for implantation and surgical fixation of neocartilage in the diseased joint.
Tissue Engineering of Immuno-Universal Cartilage
Despite widely accepted dogma of cartilage being an immuno-privileged tissue, there is strong scientific evidence that challenges this consensus. Cartilage cells (chondrocytes), just like any other cell in our body, express major histocompatibility complex (MHC) molecule type 1. This molecule is the primary factor in acceptance vs. rejection of solid organ transplants. Chondrocytes produce a crunchy matrix that surrounds them and shields them from recognition as non-self by cells of the immune system. However, with an injury to the matrix, chondrocytes that express non-self-MHC will be recognized and destroyed by the immune cells. Tissue-engineered cartilage is not an exception if manufactured from non-self-chondrocytes. Therefore, we are working on creating an immuno-universal cartilage tissue that can ‘fly under the radar’ of immune cells without being noticed as non-self.
Creating immuno-universal tissue-engineered cartilage will advance the surgeon’s ability to repair lost or diseased cartilage in human patients by using an off-the-shelf product. This solution will minimize the surgery time and the need for repeat surgery. To achieve this goal, we are working with various models. For instance, dogs that spontaneously sustain cartilage injuries can serve as a naturally occurring model where this new strategy can be explored. Pig is another model we are using for the development of immuno-universal cartilage tissue.
Contacts
Natalia Vapniarsky-Arzi
Assistant Professor
